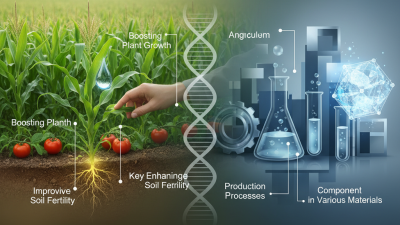
Mg Nitrate Hexahydrate is a versatile compound found in many industrial applications. Dr. Emily T. Harris, a leading expert in chemical engineering, states, “Mg Nitrate Hexahydrate is essential for agricultural and chemical sectors.” This compound plays a crucial role, particularly in fertilizers and cooling agents.
The demand for Mg Nitrate Hexahydrate is growing. Industries appreciate its effectiveness in enhancing crop yield. However, challenges remain. Not all end-users fully understand its benefits. Proper education about Mg Nitrate Hexahydrate is crucial for maximizing its potential.
Interesting applications exist beyond farming. In the chemical industry, it's widely used as a dehydrating agent. Yet, some may question its environmental impact. Responsible management is essential. While Mg Nitrate Hexahydrate offers many advantages, awareness of its use is vital for sustainability.

Magnesium nitrate hexahydrate is a hydrated form of magnesium nitrate. It is a white crystalline substance, soluble in water. This compound has a formula of Mg(NO3)2·6H2O and is widely used in various industrial applications. In agriculture, it serves as a nutrient source. Farmers use it to enhance plant growth and improve crop yields. Recent studies show that magnesium is crucial for photosynthesis.
Additionally, magnesium nitrate hexahydrate plays a vital role in the production of fertilizers. It supplies both magnesium and nitrogen, essential nutrients for plants. The global market for magnesium nitrate is projected to grow by 6.5% annually, reaching significant figures by 2026. This growth signals the increasing reliance on this compound in agriculture.
Moreover, this chemical compound serves in certain industrial processes, such as production of magnesium oxide and as a dehydrating agent. Its use in the textile industry is significant but often overlooked. However, industries face challenges when sourcing high-quality magnesium nitrate. Not all suppliers meet the required purity levels. This inconsistency can hinder production processes. Addressing these issues is essential for optimizing industrial applications.
Magnesium nitrate hexahydrate is a fascinating compound. It consists of magnesium ions, nitrate ions, and six water molecules. This structure makes it soluble in water, which is a key property for various applications. The chemical formula is Mg(NO3)2·6H2O. The hexahydrate form can be easily obtained through crystallization from a magnesium nitrate solution.
In industry, this compound plays several roles. It acts as a dehydrating agent and is vital in the production of fertilizers. Its ability to provide essential nutrients helps in enhancing plant growth. Additionally, magnesium nitrate hexahydrate is used in the production of explosives. However, handling this compound requires caution. Improper storage can lead to contamination with moisture.
Tip: Always store magnesium nitrate in a cool, dry place. This prevents degradation and preserves its quality.
Another interesting aspect is its use in pyrotechnics. It creates bright colors when burned. The compound can sometimes produce unexpected effects. For example, the residues may leave behind unwanted deposits.
Tip: Conduct small-scale tests before large applications. This helps in understanding the behavior of magnesium nitrate hexahydrate in specific conditions.
| Property | Value |
|---|---|
| Chemical Formula | Mg(NO3)2·6H2O |
| Molecular Weight | 203.30 g/mol |
| Appearance | White crystalline solid |
| Solubility in Water | Highly soluble |
| Melting Point | Decomposes above 100 °C |
| Uses in Agriculture | Fertilizer, nutrient supply |
| Uses in Industry | Used in pyrotechnics, metal treatment |
| Safety Information | Irritant to skin and eyes; handle with care |
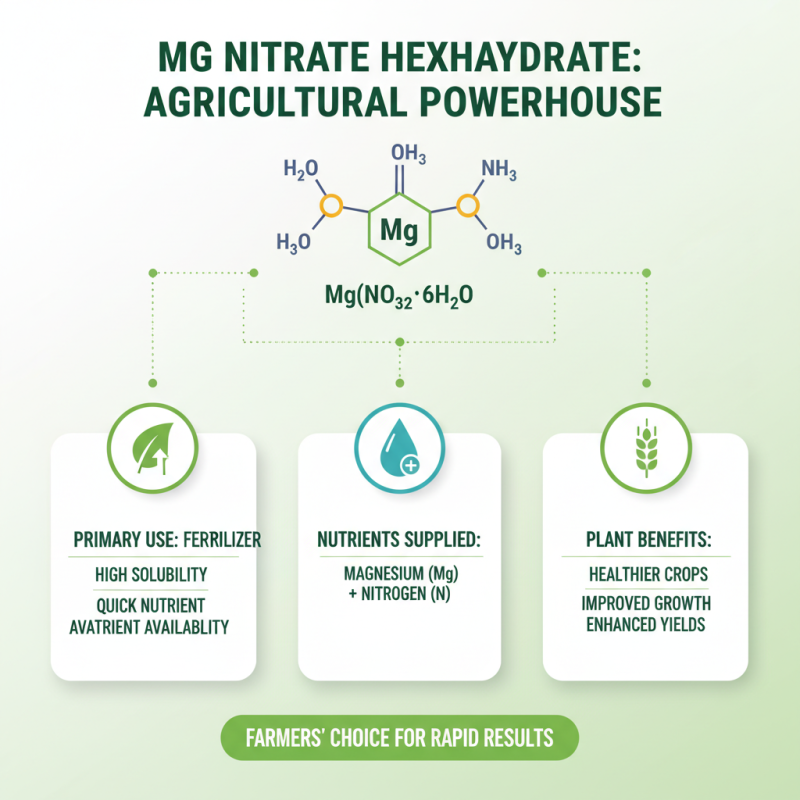
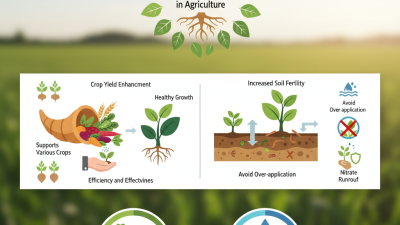
Mg Nitrate Hexahydrate is an important compound in agriculture. Its primary use lies in fertilizers. This nitrate form offers high solubility. Farmers appreciate its quick nutrient availability. Plants absorb magnesium and nitrogen readily, leading to healthier crops.
The benefits of Mg Nitrate Hexahydrate extend beyond basic nutrition. It enhances overall plant growth. It stimulates chlorophyll production. More chlorophyll means better photosynthesis. This can lead to higher yields. However, applying it correctly is crucial. Overuse can lead to nutrient imbalances.
Tip: Always conduct soil tests before applying fertilizers. This ensures your plants receive the right nutrients. The timing of application also matters. Apply during active growth phases for maximum effect.
Many farmers overlook the importance of water management. Even with the best nutrients, plants can suffer from water stress. Understanding plant needs can optimize growth. Use magnesium-rich nitrates wisely to avoid wasteful practices.
Magnesium nitrate hexahydrate is an intriguing compound with several industrial applications. In pyrotechnics and explosives manufacturing, it plays a crucial role. This compound provides oxygen, which enhances combustion efficiency. It helps produce bright flashes and colorful displays in fireworks.
The stability of magnesium nitrate hexahydrate makes it suitable for various explosive formulations. However, heat can change its properties, potentially leading to challenges during production. Correct storage conditions are vital to maintain its effectiveness. If stored improperly, the compound can absorb moisture, causing clumping and uneven performance.
Blending magnesium nitrate with other materials requires careful measurements. The chemical reactions must be precise to prevent undesirable outcomes. In practice, this means experimentation and adjustments are often necessary. This can lead to inconsistencies that require attention for successful applications. Successful use hinges on a deep understanding of its behaviors and properties over time.
Mg Nitrate Hexahydrate is widely used in various industries, known for its versatile applications. However, its industrial use raises important environmental and safety concerns. When handled improperly, this compound can have adverse effects. Workers should be aware of potential hazards.
Airborne dust is a significant issue. When Mg Nitrate Hexahydrate is transported or processed, it can create particulate matter. This can harm respiratory health. Personal protective equipment, like masks, should always be used. Spills present another risk. They require immediate attention to prevent contamination.
Proper storage is essential. Mg Nitrate Hexahydrate should be kept in cool, dry places. Containers should be tightly sealed. Inappropriate storage can lead to accidents. Regular training for staff is crucial. There should be clear protocols on handling this substance. Mistakes can lead to serious consequences. Safety data sheets must be accessible at all times. Awareness and preparedness are key to safely using this compound in industry.