Key Differences Between Calcium Ammonium Nitrate and Ammonium Nitrate

When you compare calcium ammonium nitrate to ammonium nitrate, you see clear differences in their makeup and nutrient value. Calcium Ammonium Nitrate contains both nitrogen and calcium, while ammonium nitrate only supplies nitrogen. The table below shows how each fertilizer supports soil health:
| Fertilizer Type | Nitrogen Content | Calcium Content | Benefits to Soil Health |
|---|---|---|---|
| Calcium Ammonium Nitrate | 27% | 8% | Improves soil structure, aeration, and microbial activity |
| Ammonium Nitrate | 34% | 0% | No additional benefits for soil health |
Many regions rely on calcium ammonium nitrate for crops.
- In 2022, global production reached 13.38 million metric tons.
- Europe used over 7.5 million metric tons in 2024, with France, Germany, Poland, and the UK as major consumers.
- Asia-Pacific and the Middle East & Africa also saw high usage.
Knowing these differences helps you choose the right fertilizer for your farm or garden.
Physical Properties of Calcium Ammonium Nitrate vs. Ammonium Nitrate

Appearance and Texture
When you look at calcium ammonium nitrate and ammonium nitrate, you notice differences in their granule size and texture. Calcium ammonium nitrate usually comes in larger, round granules. Ammonium nitrate has smaller, more compact granules. You can see these differences in the table below:
| Substance | Granule Size (mm) | Minimum Granule Size (%) | Bulk Density (g/cm³) |
|---|---|---|---|
| Calcium Ammonium Nitrate | 2-4 | 90.0 | N/A |
| Ammonium Nitrate | 0.5-2.5 | 90.0 | 0.76 - 0.83 |
Calcium ammonium nitrate feels less dusty and is easier to spread on fields. Ammonium nitrate can create more dust, which may cause irritation if you breathe it in. You should always wear protective gear when handling these fertilizers.
Solubility in Water
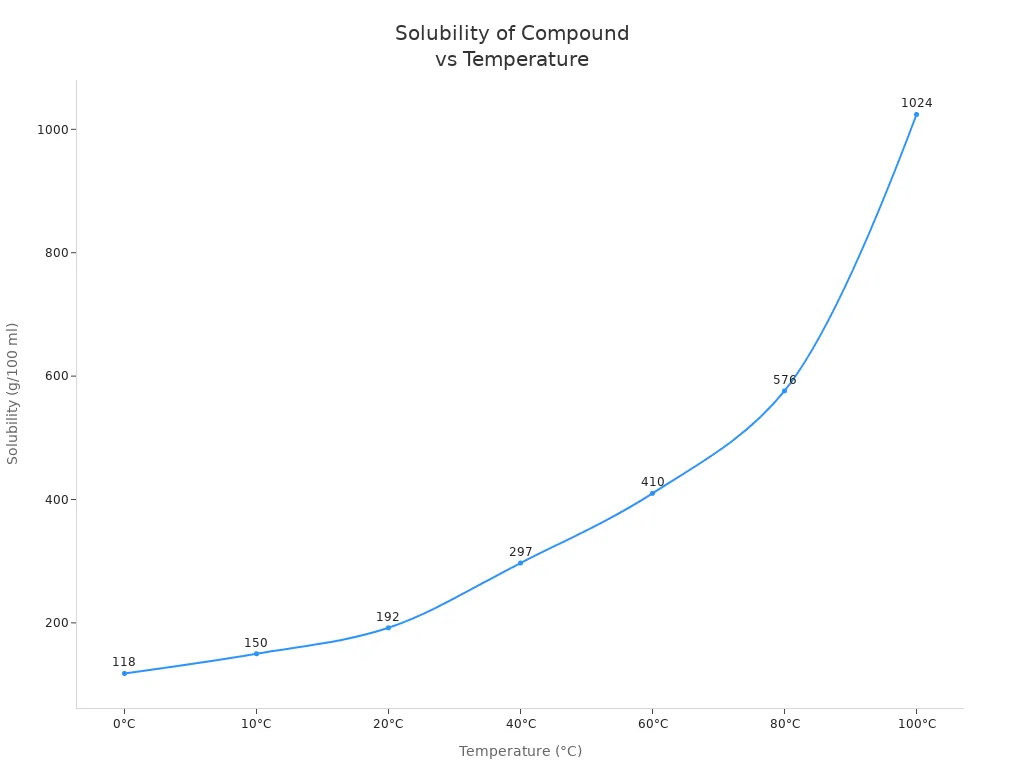
Both calcium ammonium nitrate and ammonium nitrate dissolve quickly in water. This property helps you deliver nutrients to plants through irrigation. Ammonium nitrate has a very high solubility rate, which increases as water temperature rises. The table below shows how much ammonium nitrate dissolves at different temperatures:
| Temperature (°C) | Solubility (g/100 ml) |
|---|---|
| 0 | 118 |
| 10 | 150 |
| 20 | 192 |
| 40 | 297 |
| 60 | 410 |
| 80 | 576 |
| 100 | 1024 |
You can use calcium ammonium nitrate in irrigation systems because it dissolves well and provides nutrients right away. Ammonium nitrate also dissolves fast, but it can lower soil pH and cause ammonia loss. Calcium ammonium nitrate helps keep soil pH balanced, which makes it better for many crops.
- Calcium ammonium nitrate is highly soluble, so you can use it for fertigation.
- Ammonium nitrate may cause soil acidification and ammonia loss.
- Calcium ammonium nitrate helps maintain soil pH, making it suitable for different crops.
Storage and Handling
You must store calcium ammonium nitrate and ammonium nitrate safely. Both fertilizers need dry, well-ventilated buildings. If you store ammonium nitrate, you should not keep more than 2,500 tons in one building unless you have an automatic sprinkler system. You need fire extinguishers in the warehouse and in loading areas.
Tip: Always check that your storage area has good ventilation and fire control devices.
Handling these fertilizers can be risky. Ammonium nitrate is a strong oxidizer and can explode if exposed to heat or toxic substances. You should wear protective equipment to avoid breathing in dust or fumes. Calcium ammonium nitrate can clump together in humid conditions, which makes it harder to spread. It is also an oxidizer, so you should keep it away from flammable materials.
Main handling risks:
- Ammonium nitrate can explode under certain conditions.
- It poses a fire hazard if exposed to heat or toxic substances.
- Breathing in dust or fumes can irritate your lungs and digestive system.
- Inhalation of nitrogen dioxide can cause severe lung damage.
- Calcium ammonium nitrate can clump in humid weather, making application difficult.
- Both fertilizers increase fire risk if stored with flammable items.
Agricultural Uses of Calcium Ammonium Nitrate and Ammonium Nitrate
Nitrogen Content and Release
You need to know how much nitrogen each fertilizer provides. Nitrogen helps your crops grow strong and healthy. Ammonium nitrate gives you a higher percentage of nitrogen than calcium ammonium nitrate. The table below shows the average nitrogen content for both fertilizers:
| Fertilizer Type | Nitrogen Content (%) |
|---|---|
| Ammonium Nitrate | 33.5% – 34.5% |
| Calcium Ammonium Nitrate | 26% – 28% |
Ammonium nitrate releases nitrogen quickly. Your plants can take up this nitrogen soon after you apply it. Calcium ammonium nitrate also releases nitrogen fast, but it adds calcium to the soil at the same time. This extra calcium can help your plants build stronger cell walls and improve their growth.
You should choose the fertilizer based on how much nitrogen your crops need and how quickly they need it. If you want a fast boost of nitrogen, both options work well. If your soil also needs calcium, calcium ammonium nitrate gives you an extra benefit.
Suitability for Different Crops
Different crops respond in unique ways to each fertilizer. You can see better results with calcium ammonium nitrate for some crops, especially wheat and maize. Studies show that wheat often grows better and produces higher yields when you use calcium ammonium nitrate instead of ammonium nitrate. Here are some findings from agronomic research:
- Calcium ammonium nitrate usually leads to higher crop yield and better nitrogen use efficiency, especially for wheat.
- In one study, wheat treated with calcium ammonium nitrate produced 35% more yield than wheat treated with ammonium nitrate.
- Both wheat and maize showed higher yields with calcium ammonium nitrate in the first year of a study.
- Calcium ammonium nitrate works best for wheat during early growth stages.
You should also pay attention to recommended application rates for your crops. The table below gives you a guide for maize and vegetables:
| Crop Type | Recommended Application Rate (kg per hectare) |
|---|---|
| Maize | 100-150 (split into two applications) |
| Vegetables | 50-100 (ensure even distribution) |
Tip: Split your fertilizer application for maize to give your plants a steady supply of nitrogen.
Benefits for Soil Health
You want to keep your soil healthy for future crops. The type of fertilizer you use can affect soil structure and pH. Ammonium nitrate can lower soil pH over time. This process, called soil acidification, can harm soil structure and reduce the number of helpful microbes. Long-term use of ammonium nitrate may drop soil pH by over 16% in the top layer. This change can make it harder for plants to take up nutrients.
- Long-term use of ammonium nitrate can cause soil acidification.
- Lower soil pH can hurt soil structure and reduce biodiversity.
- The nitrification process releases hydrogen ions, which leads to more acid in the soil.
Calcium ammonium nitrate helps balance soil pH because it contains calcium. This calcium can improve soil structure and support healthy microbial activity. When you use calcium ammonium nitrate, you help your soil stay healthy and productive for many years.
Environmental Impacts of Calcium Ammonium Nitrate and Ammonium Nitrate
Risk of Leaching and Runoff
You need to think about how fertilizers move through soil and water. Both calcium ammonium nitrate and ammonium nitrate can leach into groundwater if you apply them at the wrong time or in wet conditions. The timing of your fertilizer application and the type you choose make a big difference. Research shows that nitrate leaching drops by 44% when you combine dairy cattle slurry with calcium ammonium nitrate, compared to using calcium ammonium nitrate alone. This effect is strongest after the first growing season, when soil moisture is high. If you apply fertilizer before heavy rain, you increase the risk of runoff and pollution. You can reduce leaching by matching your fertilizer schedule to your crop’s needs and local weather patterns.
Effects on Soil Acidity
You want to keep your soil healthy for future crops. Fertilizers change soil pH over time. Ammonium-based fertilizers, like calcium ammonium nitrate, lower soil pH because plants release hydrogen ions when they absorb ammonium. Nitrate-based fertilizers can raise soil pH since plants release hydroxide ions when they take up nitrate. If you use ammonium-based fertilizers for many seasons, you may see a big drop in soil pH. This happens because the nitrification process creates extra hydrogen ions. Ammonium nitrate causes more acidification than calcium ammonium nitrate. Calcium in calcium ammonium nitrate helps balance the acidifying effect.
- Soil acidification happens when pH drops below 7.
- Continuous use of ammonium-based fertilizers speeds up acidification.
- Calcium in calcium ammonium nitrate helps prevent soil from becoming too acidic.
Safety and Regulations
You must follow strict rules when you store and sell fertilizers. Ammonium nitrate has more regulations because it can be dangerous if not handled properly. Many countries require special storage and limit how much you can sell. Calcium ammonium nitrate faces fewer restrictions. The table below shows how international rules differ:
| Chemical Type | Classification | Storage Requirement | Sale Requirement |
|---|---|---|---|
| Ammonium nitrate | A | ≥45% | >80% |
| Calcium ammonium nitrate | A | N/A | X |
Tip: Always check local laws before you buy or store fertilizers. Safe handling protects you and your environment.
Application Methods for Calcium Ammonium Nitrate and Ammonium Nitrate

Best Practices for Ammonium Nitrate
You can use ammonium nitrate in several ways to help your crops grow strong. Here are some of the most effective methods:
- Foliar Application: Spray a diluted solution directly onto the leaves. This method works well when plants need a quick boost, especially during fast growth or if you see signs of nutrient deficiency.
- Fertigation: Inject the solution into your irrigation system. This delivers nutrients right to the roots and helps prevent waste.
- Soil Drenching: Pour the solution around the base of your plants. This targets the root zone for efficient uptake.
To get the best results, follow these steps:
- Test your soil and plants to check nutrient levels before applying fertilizer.
- Apply ammonium nitrate at key growth stages, such as when plants are growing quickly.
- Always dilute concentrated solutions to avoid burning leaves and to spread nutrients evenly.
The timing and placement of your application matter. Split applications during the growing season can boost yields by up to 1.9 times compared to a single pre-plant application. Coarse soils lose nitrogen faster, so adjust your plan if your soil is sandy. Rainfall also helps plants absorb nitrogen, so watch the weather.
| Key Findings | Description |
|---|---|
| Timing and Placement | Split applications from V4 to V12 improved yields by 1.5 to 1.9-fold. |
| Soil Texture Impact | Coarse soils lost nitrogen quickly, reducing yield. |
| Precipitation Influence | Well-timed rain improved nitrogen uptake and fertilizer effectiveness. |
Best Practices for Calcium Ammonium Nitrate
You can apply calcium ammonium nitrate in different ways, depending on your crop. The table below shows common methods and timing:
| Crop Type | Application Method | Rate/Timing |
|---|---|---|
| Wheat | Split application | 600–750 kg/ha between stem extension and flag leaf emergence |
| Corn | Pre-plant and at 6-leaf | 40% pre-plant, 60% at 6-leaf stage |
| Tomatoes/Peppers | Pre-plant and foliar spray | 200 kg/ha pre-plant, then 0.3–0.5% solution weekly after flowering |
| Apples | Before bloom | 500–800 lbs/acre |
| Grapes | At veraison | 150 kg/ha |
For best results:
- Apply during the growing season, especially at planting and rapid growth.
- Spread granules evenly over the soil. Avoid direct contact with leaves.
- Use soil tests to decide how much to apply.
- Water after application to help nutrients reach the roots.
Tip: Split applications give your plants a steady supply of nutrients and help prevent runoff.
Efficiency and Minimizing Environmental Harm
You can protect the environment while feeding your crops. Split applications of nitrogen fertilizers help reduce nitrogen losses and balance yield with environmental impact. Studies show that split applications can lower ammonia loss by up to 80% compared to single applications.
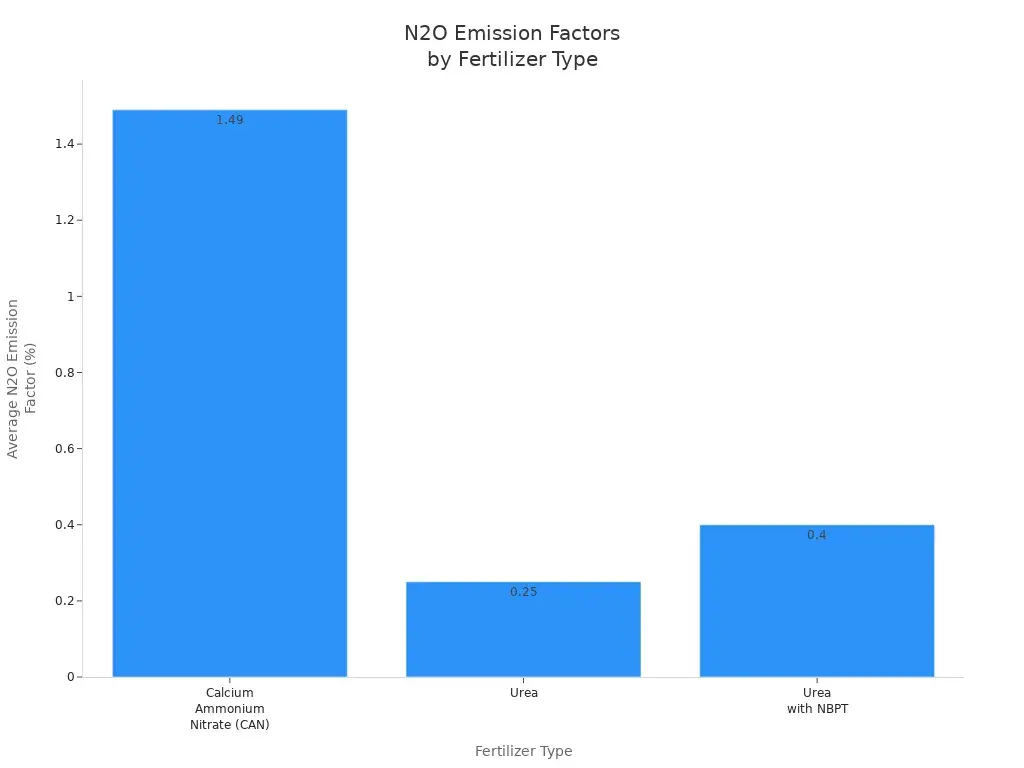
| Fertilizer Type | Average N2O Emission Factor (%) | Notes |
|---|---|---|
| Calcium Ammonium Nitrate (Can) | 1.49 | Higher emissions, variable by site |
| Urea | 0.25 | Lower emissions, less variability |
| Urea with NBPT | 0.40 | Reduces ammonia loss, effective |
Switching to urea-based fertilizers or using additives like NBPT can lower greenhouse gas emissions. You can also reduce caking and waste by choosing products made with silicic acid or calcium lignosulfonate. Always match your fertilizer plan to your soil, crop, and local weather to get the best results with the least harm.
Practical Considerations When Choosing Calcium Ammonium Nitrate or Ammonium Nitrate
Choosing Based on Soil Type
You need to match your fertilizer choice to your soil’s needs. Sandy soils lose nutrients quickly, so you may prefer calcium ammonium nitrate. It releases nutrients steadily and helps keep soil structure strong. Heavy clay soils hold nutrients longer, but they can become compacted. Calcium in CAN improves aeration and supports root growth. If your soil tests show low pH, CAN helps balance acidity. Ammonium nitrate works best in soils with neutral pH and good drainage. You should always test your soil before deciding.
Tip: Use soil tests to check pH and nutrient levels. This helps you pick the right fertilizer for healthy crops.
Climate and Weather Factors
Weather affects how fertilizers work in your fields. You want a product that performs well in your local climate. CAN works in both dry and wet climates. It does not absorb moisture from the air easily, so it stays free-flowing and does not cake. This means you can spread it evenly, even when humidity is high. CAN’s balanced nutrients help crops grow fast and keep nutrients in the soil longer. You get better yields in changing weather.
- CAN stays stable in humid conditions and does not clump.
- CAN supports quick nutrient uptake and steady growth in all climates.
Ammonium nitrate can absorb water from the air and form lumps. This makes it harder to spread in wet weather. You may see uneven growth if nutrients do not reach all plants.
Long-Term Farm Management
You want to think about the future of your farm. Fertilizer choice affects your land, your costs, and the environment. CAN can cause nitrogen runoff if you use too much. This runoff can pollute water and harm fish and plants. Many countries, like those in the European Union, have strict rules for nitrogen fertilizers to protect nature. Farmers now look for safer options and new technologies.
| Evidence | Description |
|---|---|
| Environmental Concerns | Too much CAN can pollute water and harm aquatic life. |
| Regulatory Measures | The EU has strict rules to limit nitrogen pollution. |
| Market Dynamics | Environmental risks slow CAN market growth as farmers seek alternatives. |
| Slow-Release Fertilizers | New products help reduce nutrient loss and protect the environment. |
| Demand for Advanced Tech | Farmers want better CAN products for sustainable farming. |
You can choose slow-release fertilizers to lower nutrient loss and protect your soil. As farming changes, you may see more advanced CAN products that help you grow healthy crops and care for the environment.
You see clear differences between calcium ammonium nitrate and ammonium nitrate. Calcium ammonium nitrate gives you both nitrogen and calcium, which helps your soil stay healthy. Ammonium nitrate supplies only nitrogen. You should match your fertilizer to your crop and soil needs.
- Test your soil before you choose.
- Watch your local weather for best results.
Tip: Pick the fertilizer that supports your farm’s long-term health and yield.
FAQ
What is the main difference between calcium ammonium nitrate and ammonium nitrate?
You get both nitrogen and calcium from calcium ammonium nitrate. Ammonium nitrate gives you only nitrogen. Calcium helps your soil stay healthy and supports strong plant growth.
Can you use both fertilizers for all crops?
You can use both for many crops. Calcium ammonium nitrate works best for wheat, maize, and vegetables. Ammonium nitrate suits crops that need fast nitrogen, like corn and grass.
Is ammonium nitrate safe to store at home?
You must store ammonium nitrate carefully. It can explode if exposed to heat or fire. Always keep it in a cool, dry place away from flammable items.
Tip: Use protective gear when handling ammonium nitrate to stay safe.
Does calcium ammonium nitrate help prevent soil acidity?
You help your soil stay balanced when you use calcium ammonium nitrate. The calcium in it keeps soil pH steady and supports healthy microbes.
| Fertilizer Type | Effect on Soil pH |
|---|---|
| Calcium Ammonium Nitrate | Balances and protects |
| Ammonium Nitrate | Can lower pH |
Which fertilizer is better for humid climates?
You get better results with calcium ammonium nitrate in humid weather. It does not clump or cake, so you can spread it easily. Ammonium nitrate may form lumps and become hard to use.