Nitrocellulose: The Revolutionary Material from Explosives to Plastics

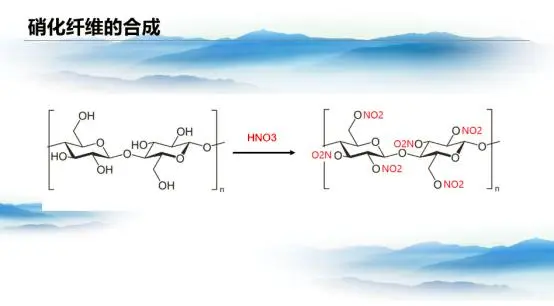
The advent of nitrocellulose marked a major breakthrough in the field of cellulose-based materials. In 1832, French chemist Henri Braconnot first synthesized nitrocellulose by treating wood and cotton with concentrated nitric acid. In 1846, Christian Friedrich Schönbein refined the process by using a mixture of Nitric Acid And sulfuric acids, significantly improving reaction efficiency. Chemically, this process is an esterification reaction—hydroxyl groups in cellulose react with nitric acid to form cellulose nitrate, releasing water in the process. The addition of sulfuric acid effectively absorbs this water, enhancing the degree of nitration. As nitration increases, so does the flammability and explosiveness of nitrocellulose, making it an ideal candidate for explosives.
The instability of nitro compounds lies at the heart of their explosive nature. When heated, they decompose to release oxygen, while the carbon within the molecule acts as fuel, enabling combustion without external oxygen. This process generates intense heat, accelerating further decomposition and creating a chain reaction. The combustion products—carbon dioxide, water, and nitrogen gas—cause a rapid expansion of gases, making nitro compounds highly flammable and explosive.
Nitrocellulose, known as "guncotton" for its remarkable explosive power, boasted a detonation force two to three times greater than traditional black powder, with the added advantage of producing no smoke. This made it the preferred choice for smokeless propellants. However, its extreme hazard prompted scientists to seek safer alternatives, eventually leading to Alfred Nobel’s development of stabilized explosives, which replaced nitrocellulose in many applications.
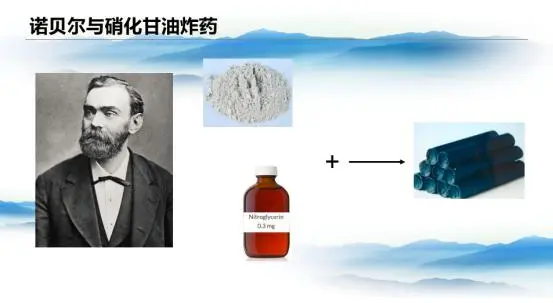
Nobel’s contributions extended far beyond this. By adsorbing nitroglycerin onto diatomaceous earth, he dramatically improved its safety. Nitroglycerin, first synthesized in 1847, was notoriously sensitive to shock and difficult to handle—until 1879, when its medicinal use for treating angina was discovered. Nobel’s innovation required a detonator to ignite nitroglycerin, greatly reducing accidental risks.
The significance of nitrocellulose was not limited to explosives; it also paved the way for two other groundbreaking inventions: celluloid and rayon. Celluloid, the first human-made plastic, emerged from a serendipitous discovery. When guncotton was dissolved in ether and evaporated, it formed a gelatinous substance known as collodion, initially used as a wound dressing akin to modern bandages. Later, American inventor John Wesley Hyatt found that adding camphor significantly enhanced the hardness and flexibility of collodion, giving birth to celluloid. The name "Celluloid" derives from "cellulose," reflecting its origin.
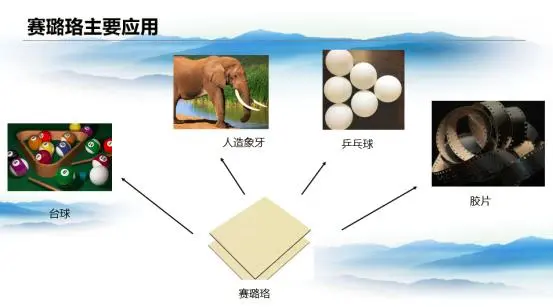
With its ivory-like texture, celluloid was widely used in billiard balls, knife handles, photographic film, and table tennis balls. Although it was made from low-nitrogen nitrocellulose and less flammable than its explosive counterpart, it still posed safety risks. As safer synthetic plastics emerged, celluloid gradually faded from everyday use.
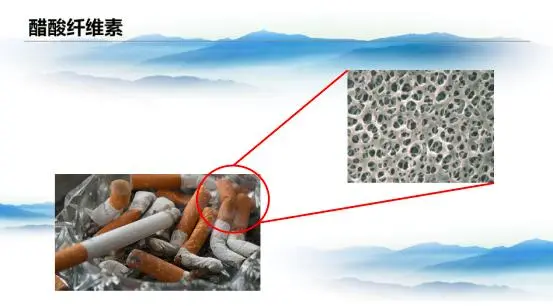
Another milestone in cellulose modification was cellulose acetate. First synthesized in 1869, it wasn’t until 1924 that it entered industrial production. While its applications were relatively limited, cellulose acetate became a key material in cigarette filters and membrane separation technology.
The story of nitrocellulose is not just a tale of scientific discovery—it is a testament to human ingenuity. From battlefields to everyday life, its legacy endures, showcasing the remarkable evolution of materials science.
Nitric acid serves as a fundamental raw material in the chemical synthesis of nitrocellulose, nitroglycerin, celluloid, and cellulose acetate. The industrial-grade nitric acid produced by Henan Yongchang Nitro Fertilizer Co. Ltd finds wide application in the manufacturing of these products.